27 Feb Review of EKB 2024: Short stem issues are huge
Newsletter February 28, 2024
Welcome!
Dear ladies and gentlemen, dear customers and interested parties,
I am pleased to introduce you to the current newsletter from ARTIQO GmbH.
Immerse yourself with us in the latest results relating to short-stem endoprosthetics, which were discussed in two exciting events as part of the EKB 2024.
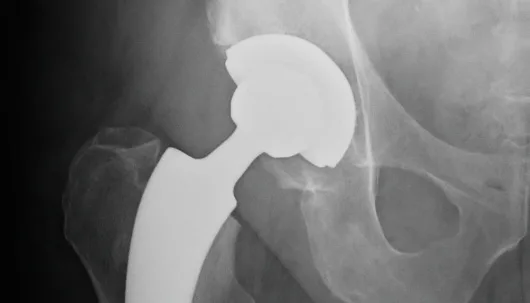
We have a whole range of news for you about our A2® short stem system: The cement-free A2® short stem (types B and G) was recently rated very well by the British Orthopedic Data Evaluation Panel (ODEP). You can find the details in our article.
And that is also a reason for us to be happy: The RSA migration analysis of the A2® short stem system is now available as a publication. We will inform you about the special features of the study, which provides reliable information about the long-term prognosis and safety of the implant.
Last but not least: The first MDR product certificates for the A2® short stem system and the 4-motion® knee system can be downloaded from the ARTIQO website. We made the preparations for the MDR re-certification very early on, so that the first certificates are already available.
As always, we hope you enjoy reading!
Yours, Ulrich Bücken
ARTIQO GmbH
Short stem supply:
A concept of the future
The German Endoprosthesis Register (EPRD) has been reporting increases in short stem care for years. Most recently, the proportion of short stems was 13.3%. Nevertheless, the short stem often falls short, according to the opening statement by Prof. Dr. Stefan Budde, Bielefeld, and Dr. Sebastian Meller, Berlin. The two scientific directors of the EKB Short Stem Seminar and the invited experts are therefore united in the goal of advancing the topic of short stems across manufacturers and implants.
EKB 2024:
A system for all cases
The speakers Professor Dr. Stefan Budde, Bielefeld, and Dr. Ahmed Yaseen, Innsbruck, discussed with the participants of the ARTIQO workshop as part of the Endoprosthetics Congress Berlin 2024 how the A2® short stem system can become a system for all cases.
5A* ODEP rating for the cementless A2® short stem
There is reason to be happy at ARTIQO these days. The British Orthopedic Data Evaluation Panel (ODEP) has awarded the cement-free A2® short stem (types B and G) a 5A* rating. This highest possible rating after 5 years of service is based on a validated database and is awarded by an independent evaluation committee according to defined criteria.
Would you like to find out more about ARTIQO’s company anniversary, milestones and product developments? Then follow us on LinkedIn.
RSA migration analysis for the cementless A2® stem published
The latest issue of Scientific Report contains the randomized RSA migration analysis of the A2® short stem system. It makes important statements about the long-term prognosis and safety of the implant. We summarize some key aspects of the study for you.
First MDR product certificates are available for download
The first MDR product certificates – more precisely the “EU certificate for the assessment of the technical documentation” – are available for the implants of the A2® short stem system and the 4-motion® knee system. They are valid until the end of 2028 and are available to our customers for download on the ARTIQO website.